X-Pure GelMA
What is X-Pure GelMA?
X-Pure® GelMA is the world’s first gelatin methacryloyl (GelMA or GelMOD) produced under GMP conditions1, making it suitable for clinical applications in advanced biomedical applications.
This premium biomaterial is ideal for 3D bioprinting, regenerative medicine and tissue engineering owing to its guaranteed ultra-low impurity levels, batch to batch consistency and tunable mechanical properties. As a result, X-Pure GelMA can reduce the development time between bench and bedside, for pharmaceuticals, medical devices and Advanced Therapy Medicinal Products (ATMP).
Rousselot X-Pure GelMA portfolio
| Product | MW (kDa) | DoM (%) | Endotoxin Level (EU/g) | MA (ppm) | Research Grade | GMP grade |
|---|---|---|---|---|---|---|
| X-Pure GelMA 160P40 | 160 | 40 | <10 | <30 | 1-100g | Available upon request |
| X-Pure GelMA 160P60 | 60 | <10 | <30 | |||
| X-Pure GelMA 160P80 | 80 | <10 | <30 | |||
| X-Pure GelMA 90P40 | 90 | 40 | <10 | <30 | ||
| X-Pure GelMA 90P60 | 60 | <10 | <30 | |||
| X-Pure GelMA 90P80 | 80 | <10 | <30 | |||
| X-Pure GelMA custom | Custom | Custom | <10 | <30 |
How X-Pure GelMA works
A GelMA hydrogel allows the formation of 3D architectures that are stable at body temperature. X-Pure GelMA rheological properties can be customized by varying the degree of modification (the number of crosslinks between gelatin protein chains) and the gelatin molecular weight, making it one of the most versatile hydrogels available for 3D cell culture and drug delivery systems2.
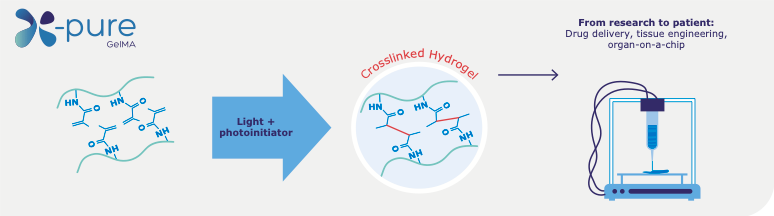
Figure 1. The methacrylamide side groups are photo crosslinked in the presence of a photo initiator and UV or visible light, creating a viscoelastic gelatin network. The degree of modification (number of methacrylamide side groups) and molecular weight can be tuned for different biomedical applications.
Watch our video to find out how GelMA is made and how it can be used for tissue engineering, drug delivery & other applications.
Applications of X-Pure GelMA
With ultra-low endotoxin levels, Rousselot’s X-Pure GelMA is suitable for the most sensitive biomedical & pharmaceutical applications, such as:
- 3D bioprinting
- Tissue engineering
- 3D cell culturing
- Regenerative medicine (preclinial and clinical)
- Medical devices
- Advanced therapy medicinal products (ATMP)
View our customer product pipeline to see the medical devices and pharmaceuticals currently in development using X-Pure products.
Advantages of X-Pure GelMA
Rousselot’s X-Pure GelMA stands out by offering several unique advantages.
High batch-to-batch consistency
The X-Pure GelMA standardized production process allows for high reproducibility and controllability in terms of composition and biophysiochemical properties. Lower material variability means a higher signal-to-noise ratio for your trials.
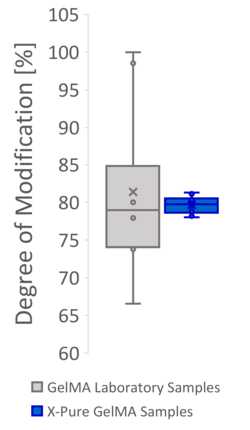
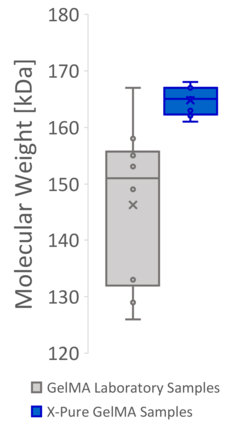
Figure 2: Producing GelMA at the lab scale based on processes described in the literature often results in products with a high variability in methacryloyl functionalization and molecular weight, causing variable mechanical properties. This can mainly be attributed to the lack of process controls at lab scale. The same variability however is also seen in many commercial GelMA products, which use a similar process.
Find out more in our video: No more batch lottery when making a new GelMA
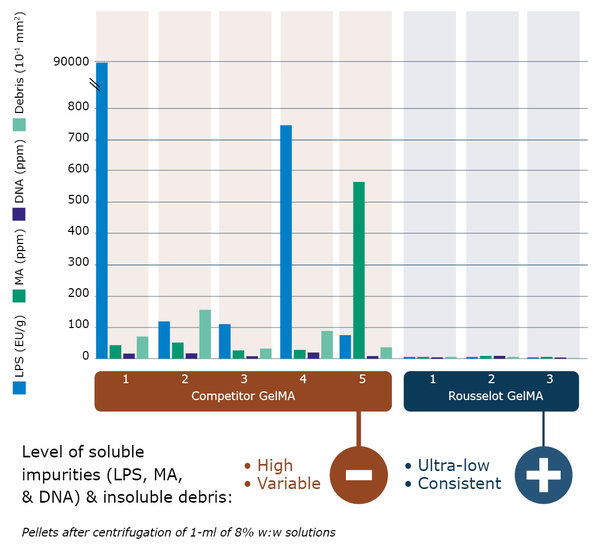
Figure 3: Analysis of commercially available GelMA products shows a high and variable level of soluble impurities such as LPS, Methacrylic acid (MA) and DNA, and even insoluble debris in all products (pellets after centrifugation of 1-ml of 8% w:w solutions).
Find out more in our video: X-Pure - Bridging the valley of death
GMP grade & scalable production process
X-Pure GelMA is the first commercially available GMP GelMA. X-Pure Research grade and X-Pure GMP grade are functional equivalence products, meaning requalification efforts when switching to the GMP product can be avoided. This can accelerate translation from lab to clinic.
To find out more about GMP grade GelMA, click here.
Reach out to our expert team for more info on co-innovation or formulation.
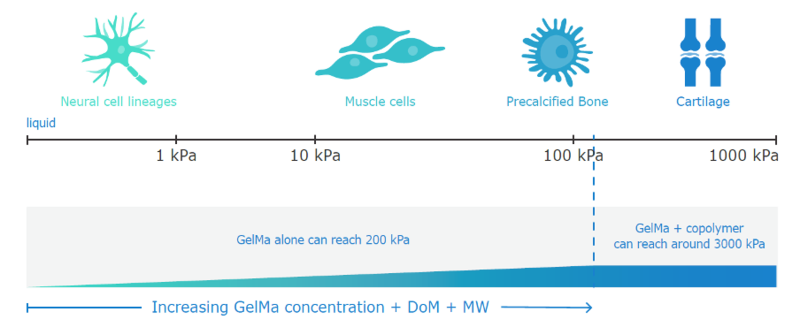
Figure 4: Graph to show how different cells require different environments
References:
[1] IPEC. Excipient Good Manufacturing Practices Guide, 2022
[2] Pepelanova, I., Kruppa, K., Scheper, T. and Lavrentieva, A., 2018. Gelatin-Methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering, 5(3), p.55